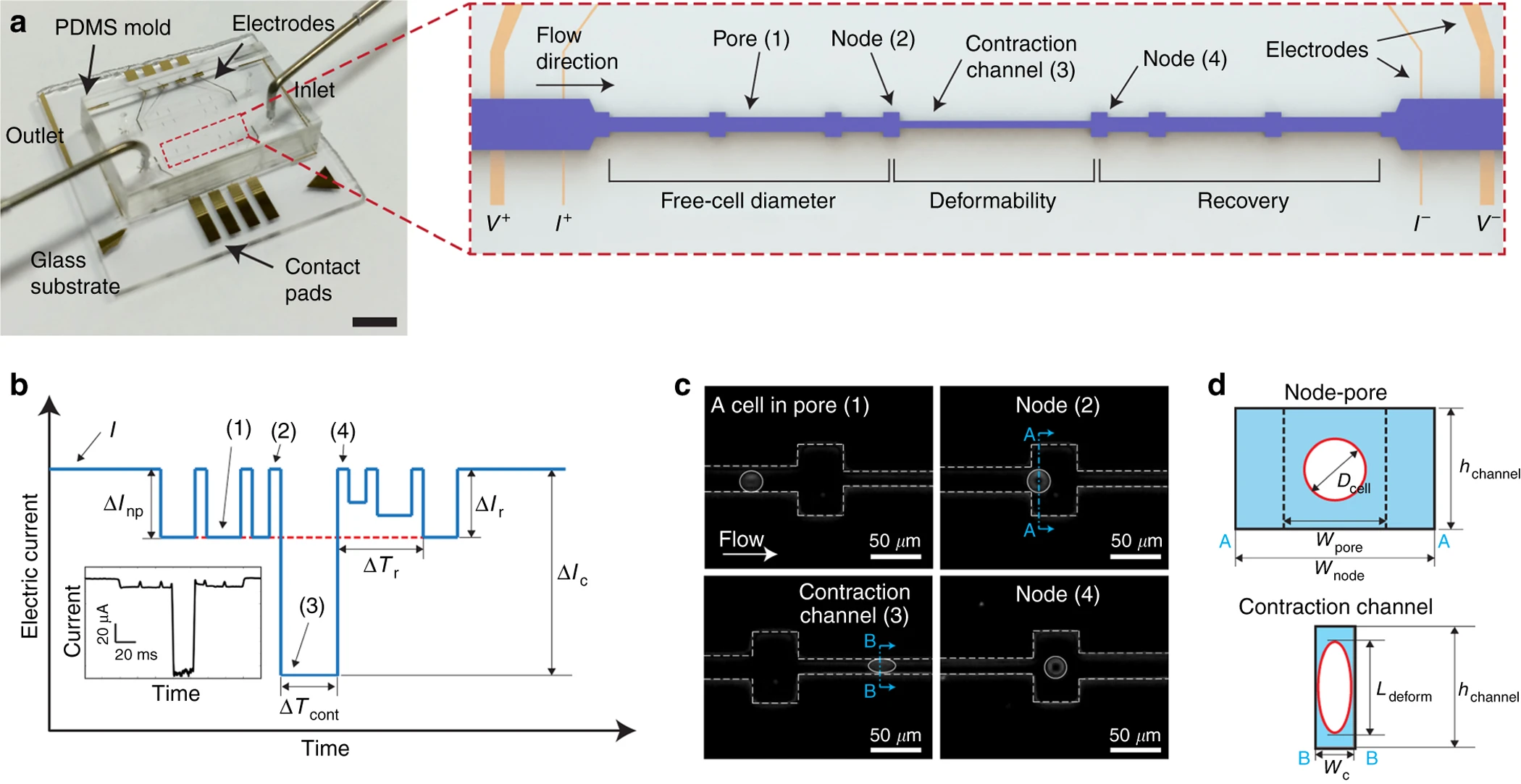
Featured Publications
All Publications
S. Hinz, S. M. Grondal, M. Miyano, J. C. Lopez, K. L. Cotner, T. Taylor, C. Chen, E. J. Hester, V. E. Seewaldt, J. B. Lorens, L. L. Sohn, and M. A. LaBarge, Identifying Individuals Susceptible to Breast Cancer Based on Mechanical Properties of Single Cells, submitted, 2025.
- C. Pierce, K. Suryoraharjo, I. H. Robertson, X. Su, D. B. Hatchett, A. Shin, K. N. Adams, E. Berthier, S. Thongpang, A. Ogata, A. B. Theberge, and L. L. Sohn, CandyCollect: An Open-Microfluidic Device for the Direct Capture and Enumeration of Salivary-Extracellular Vesicles, bioRxiv (2024). doi: 10.1101/2024.10.09.617508
- S. T. Mahmud, W. Mao, T. Thomsen, C. Chen, R. Rex, A. Lai, L. L. Sohn, and A. Randles, Microfluidic Digital Twin for Enhanced Single-Cell Analysis, accepted to the International Conference on Computation Science (2025)
- A. Lai*, S. Hinz*, A. Dong, M. Lustig, M. A. LaBarge, and L. L. Sohn, Multi-Zone Visco-Node-Pore Sensing: A Microfluidic Platform for Multi-Frequency Viscoelastic Phenotyping of Single Cells, Adv. Sci. 2024 Sep23:e2406013. doi: 10.1002/advs.202406013. (*) denotes equal contribution.
A. Dong, R. Rex, J. Chen, L. L. Sohn, and M. Lustig, Metal Pad Sensing: Exploiting the Electric Double Layer to Improve Resistance-Based Microfluidic Cell Tracking, with Applications to Label-free Mechanophenotyping, submitted, 2024.
- R. Rex, E. Petit, and L. L. Sohn, Cell-Softening Node-Pore Sensing Enables Rapid and Sensitive Strain-Dependent Measurement of Cellular Viscosity, submitted (2024).
- E. Lindberg, T. Wu, K. Cotner, A. Glazer, A. Jamali, L. L. Sohn, T. Alliston, G. D. O’Connell, Priming Chondrocytes During Expansion Alters Cell Behavior and Improves Matrix Production in 3D Culture, accepted to Osteoarthritis and Cartilage (2023). doi: 10.1016/j.joca.2023.12.006.
- A. Dong, L. L. Sohn, M. Lustig, Metal Pad-Enhanced Resistive Pulse Sensor Reveals Complex-Valued Braess Paradox, Phys. Rev. E 108, 014408 (2023) doi: 10.1103/PhysRevE.108.014408.
- C. Lu, R. Rex, Z. Lung, J. Wang, F. Wu, H. J. Kim, L. Zhang, L. L. Sohn, and A. Dernburg, A cooperative network at the nuclear envelope counteracts LINC-mediated forces during oogenesis in C. elegans, Sci. Adv. 2023 Jul 14;9(28):eabn5709. doi: 10.1126/sciadv.abn5709.
- A. Lai*, R. Rex*, K. Cotner, A. Dong, M. Lustig, and L. L. Sohn, Mechano-node-pore sensing: a rapid, label-free platform for single-cell viscoelastic measurements, JoVE, published 2022 Dec 2. (*) denotes equal contribution. doi: 10.3791/64665
- T. R. Carey, M. Kozminsky, J. Hall, V. Vargas-Zapata, K. Geiger, L. Coscoy, and L. L. Sohn, Detecting Intact Virus Using Exogeneous Oligonucleotide Labels, Anal. Chem. 2022 May 31;94(21):7619-7627. doi.org/10.1021/acs.analchem.2c00835
- B. Li, A. Maslan, S. Kitayama, C. Pierce, A. Streets, and L. L. Sohn, Mechanical phenotyping of acute promyelocytic leukemia reveals unique biomechanical responses in retinoic acid-resistant populations, iScience. 2022 Feb 18. doi.org/10.1016/j.isci.2022.103772
- B. Li*, K. Cotner*, N. K. Liu, S. Hinz, M. A. LaBarge, and L. L. Sohn, Evaluating Sources of Technical Variability in the Mechano-Node-Pore Sensing Pipeline and Their Effect on the Reproducibility of Single-Cell Mechanical Phenotyping, PLoS One. 2021 Oct 25. (*) denotes equal contribution. doi.org/10.1371/journal.pone.0258982
- M. Kozminsky, T. R. Carey, and L. L. Sohn, DNA-Directed Patterning for Versatile Validation and Characterization of a Lipid-Based Nanoparticle Model of SARS-CoV-2, Adv Sci. 2021 Oct 21. doi.org/10.1002/advs.202101166
- M. Kozminsky*, O. Scheideler*, B. Li, N. Liu, and L. L. Sohn, Multiplexed DNA-Directed Patterning of Antibodies for Applications in Cell Subpopulation Analysis, ACS Apply Mater Interfaces. 2021 Oct 6. (*) denotes equal contribution. doi.org/10.1021/acsami.1c15047
- S. Hinz, A. Manousopoulou, M. Miyano, R. W. Sayaman, K. Y. Aguilera, M. E. Todhunter, J. C. Lopez, L. L. Sohn, L. D. Wang, and M. A. LaBarge, Deep Proteome Profiling of Human Mammary Epithelia at Lineage and Age Resolution, iScience. September 24, 2021. doi.org/10.1016/j.isci.2021.103026
- K. A. Cabral, D. M. Patterson, O. J. Scheideler, R. Cole, A. R. Abate, D. V. Schaffer, L. L. Sohn, and Z. J. Gartner, Simple, affordable, and modular patterning of cells using DNA programmed assembly of cells, J. Vis. Exp. 2021 Feb 24. dx.doi.org/10.3791/61937-v
- L. L. Sohn, P. Schwille, A. Hierlemann, S. Tay, J. Samitier, J. Fu, P. Loskill, How can microfluidic and microfabrication approaches make experiments more physiologically relevant?, Cell Syst. 2020 Aug 29. doi.org/10.1016/j.cels.2020.07.003
- M. Kozminsky and L. L. Sohn, The promise of single-cell mechanophenotyping for clinical applications, Invited Perspective, Biomicrofluidics. 2020 Jun 9. https://doi.org/10.1063/5.0010800 Selected as a Feature Article.
- O. Scheideler, C. Yang, M. Kozminsky, K. I. Mosher, R. Falcon-Banchs, E. C. Ciminelli, A. W. Bremer, S. Chern, D. V. Schaffer, and L. L. Sohn, Recapitulating complex biological signaling environments using a multiplexed, DNA-patterning approach. Science Advances. 2020 Mar 18. doi.org/10.1126/sciadv.aay5696
- T. Carey, B. Li, and L. L. Sohn, Using Node-Pore Sensing for Characterizing Cells and Extracellular Vesicles, Invited Chapter in Methods in Molecular Biology, eds. M. Ossandon and A. Rasooly, Humana Press (2019).
- M. Muncie, R. Falcon-Banchs, J. N. Lakins, L. L. Sohn, and V. M. Weaver, Patterning the geometry of human embryonic stem cell colonies on compliant substrates to control tissue-level mechanics, J. Vis. Exp. (151), e60334, doi:10.3791/60334 (2019).
- J. Kim, B. Li*, O. J. Scheideler*, Y. Kim, and L. L. Sohn, Visco-Node-Pore Sensing: A Microfluidic Rheology Platform to Characterize Viscoelastic Properties of Epithelial Cells, iScience 13, 214-228 (2019), doi: 10.1016/j.isci.2019.02.021. (*) denotes equal contribution.
- H. Jiang, L. L. Sohn, H. Huang, and L. Chen, Single Cell Clustering Based on Cell-Pair Differentiability Correlation and Variance Analysis, Bioinformatics 2018 May 16. doi: 10.1093/bioinformatics/bty390.
- T. Carey, K. Cotner, B. Li, and L. L. Sohn, Developments in label-free microfluidic methods for single-cell analysis and sorting, Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018 Apr 24:e1529. doi: 10.1002/wnan.1529 Top Downloaded Article for WIREs Nanomedicine and Nanobiotechnology in 2019.
- S. Han, J. Kim, R. Li, A. Ma, V. Kwan, K. Luong, and L. L. Sohn, Hydrophobic patterning-based 3D microfluidic cell culture assay, Adv Healthc Mater 2018 Jun; 7(12):e1800122. doi: 10.1002/adhm.201800122.
- M. Kellman, F. Rivest, A. Pecachek, L. L. Sohn, and M. Lustig, Node-Pore Coded Coincidence Correction: Coulter Counters, Code Design, and Sparse Deconvolution, IEEE Sensors Journal 8, 3068-3079 (2018). 10.1109/JSEN.2018.2805865.
- J. Kim, S. Han, A. Lei, M. Miyano, J. Bloom, V. Srivastava, M. R. Stampfer, Z. J. Gartner, M. A. LaBarge, and L. L. Sohn, Characterizing cellular mechanical phenotypes with mechano-node-pore sensing, accepted September 16, 2017 to Nature Microsystems & Nanoengineering. Published online March 2018, doi: 10.1038/micronano.2017.91. Featured article and highlighted on the homepage of Nature Microsystems & Nanoengineering (March 12, 2018).
- Y. Kim, J. Kim, O. Scheideler, E. Cimenelli, and L. L. Sohn, Microfluidic Rheology to Study Effects fo Cell Cycle to Viscoelastic Properties of Epithelial Cells, Biophysical Journal 114, 541a (2018).
- B. Li, J. Kim, and L. L. Sohn, Mechanical Phenotyping of Acute Myeloid Leukemias for Predicting Response to Retinoic Acid, Biophysical Journal 114, 541a (2018).
- Schiffman, C. Lin, F. Shi, L. Chen, L. L. Sohn, and H. Huang, Clustering analysis for single–cell RNA–sequencing data with SIDEseq: a cell similarity measure defined by shared identified differentially expressed genes, Statistics in Biosciences 2017, vol. 9, issue 1, No 11, 200-216.
- Falcon-Banchs, F. Rivest, and L. L. Sohn, Single-Cell Label-Free Profiling, Encyclopedia of Analytical Chemistry, D. Pappas (ed.), Wiley, United Kingdom, published online March 25, 2017. https://doi.org/10.1002/9780470027318.a9565.
- S. Han, Y. Shin, H. Jeong, J. S. Jeon, R. D. Kamm, D. Huh, L. L. Sohn, and S. Cheong, Constructive remodeling of a synthetic endothelial extracellular matrix, Scientific Reports 5, Article No. 18290, published online 21 December, 2015. doi: 10.1038/srep18290.
- R. Rivest, A. P. Pechacek, R. Park, K. Goodman, N. Cho, M. Lustig, and L. L. Sohn, Toward real-time cell detection and characterization using Barker-coded Node-Pore Sensing, µTAS 2015 Conference Proceedings (2015).
- D. Yang, S. Leong, and L. L. Sohn, High-throughput microfluidic device for circulating tumor cell isolation from whole blood, µTAS 2015 Conference Proceedings, 2015.
- J. Kim, A. Lei, and L. L. Sohn, Characterizing mechanical properties of cancer cells by Node-Pore Sensing, µTAS 2015 Conference Proceedings, 2015. Finalist for Best Poster Award
- D. Yang, S. Leong, A. Lei, and L. L. Sohn, High-throughput microfluidic device for rare cell isolation, Proc. SPIE 9518, Bio-MEMS and Medical Microdevices II, 95180E (1 June 2015); doi: 10.1117/12.2178613.
- O. J. Scheideler, L. L. Sohn, and D. V. Schaffer, Emerging Engineering Strategies for Studying the Stem Cell Niche, to appear in Volume 1: Biology of Stem Cell Niche, Springer’s Stem Cells and Regenerative Medicine book series (2015).
- K. Balakrishnan, J. Whang, R. Hwang, J. Hack, L. Godley, and L. L. Sohn, Node-pore sensing enables label-free surface-marker profiling of single cells, Analytical Chemistry, DOI: 10.1021/ac504613b. Selected by the Scientific Editors of the journals belonging to the American Chemical Society to be featured in “ACS Editors’ Choice”, for “research that exemplifies the Society’s commitment to improving people’s lives through the transforming power of chemistry” and for “broad public interest”.
- E. Jabart, S. Rangarajan, C. Lieu, J. Hack, I. Conboy, and L. L. Sohn, A Microfluidic Method for the Selection of Undifferentiated Human Embryonic Stem Cells and In Situ Analysis, Microfluid Nanofluid, DOI: 10.1007/s10404-014-1485-9 (2014).
- M. Mir, O. Scheideler, J. Whang, and L. L. Sohn, A Simple Optofluidic Platform for Label-Free Cell-Surface Marker Screening, Proc. SPIE 9155, Translational Biophotonics 91551C, DOI: 10.1117/12.2057806 (June, 2014).
- E. Jabart, K. Balakrishnan, L. L. Sohn, Microfluidic Techniques to Isolate and Screen Single Stem Cells, in Stem Cells and Tissue Engineering, 2nd Edition, Eds. S. Li, World Scientific Publishing, In press.
- M. R. Chapman, K. Balakrishnan, J. Li, M. J. Conboy, H. Huang, S. K. Mohanty, E. Jabart, J. Hack, I. M. Conboy, and L. L. Sohn, “Sorting single satellite cells from individual myofibers reveals heterogeneity in cell-surface markers and myogenic capacity,” Integrative Biology 2013, 5(4) 692-702.
- K. Balakrishnan, G. Anwar, M. R. Chapman, T. Ngyuen, A. Kesavaraju, and L. L. Sohn, “Node-Pore Sensing: A Robust, High-Dynamic Range Method for Detecting Biological Species,” Lab Chip 2013, 13, 1302-1307. Selected by the Editors to be in the themed category, “Lab on a Chip Top 10%,” for being of “exceptional significance for the miniturisation community” and demonstrating a “breakthrough in device technology [and] methodology”.
- Karthik Balakrishnan and Lydia L. Sohn, “Cell Screening Using Resistive-Pulse Sensing,” in Methods in Cell Biology 112, Ed. P. Michael Conn, Elsevier, pp. 369-387 (2012).
- L. L. Sohn, “Fields, Forces, and Flows in Biological Systems, by Alan J. Grodzinsky—A Review”, Quarterly Review of Biology 87, 159 (2012).
- K. Balakrishnan, M. R. Chapman, A. Kesavaraju, and L. L. Sohn, “A variable cross-section pore for screening cells for specific markers,” in Nanopores for Bioanalytical Applications: Proceedings of the International Conference, Eds. J. Edel and T. Albrecht, RSC Publishing, pp. 18-23 (2012).
- M. R. Chapman, K. Balakrishnan, M. J. Conboy, S. K. Mohanty, E. Jabart, J. Li, H. Huang, J. Hack, I. M. Conboy, and L. L. Sohn, “Label-free screening of niche-to-niche variation in Satellite stem cells using functionalized pores,” in Nanopores for Bioanalytical Applications: Proceedings of the International Conference, Eds. J. Edel and T. Albrecht, RSC Publishing, pp. 38-42 (2012).
- M. R. Chapman and L. L. Sohn, “Label-Free Resistive Pulse Cytometry,” Methods in Cell Biology: Cytometry Vth Edition, Recent Advances in Cytometry 102 Eds. Z. Darzynkiewicz, E. Holden, A. Orfao, W. G. Telford, and D. Wlodkowic. Elsevier, pp. 127-57 (2011).
- R. Dylla-Spears, J. E. Townsend, L. Jen-Jacobson, L. L. Sohn, and S. J. Muller, “Single-Molecule Sequence Detection via Microfluidic Planar Extensional Flow at a Stagnation Point,” Lab Chip 2010 Jun 21;10(12):1543-9. Epub 2010 Mar 31.
- R. Dylla-Spears, J. E. Townsend, L. Jen-Jacobson, L. L. Sohn, and S. J. Muller, “Single-Molecule Sequence Detection via Microfluidic Planar Extensional Flow at a Stagnation Point”, submitted to Lab Chip. (2009).
- R. Dylla-Spears, J. E. Townsend, L. L. Sohn, L. Jen-Jacobson, and S. J. Muller, “Fluorescent Marker for Direct Detection of Specific dsDNA Sequences”, Anal. Chem. 81, 10049-10054 (2009).
- S. K. Mohanty, M. Conboy, I. Conboy, and L. L. Sohn, “Stem-Cell Surface Marker Interrogation via Resistive-Pulse Sensing: Screening for Sca-1 Expression in Mouse Muscle Stem Cells”, The Proceedings of µTAS 2009 Conference, Korea (2009).
- O. A. Saleh and L. L. Sohn, “Resistive-Pulse Sensing and On-Chip Artificial Pores for Biological Sensing”, Invited Chapter in Nano and MicroSensors for Chemical and Biological Surveillance, eds. J. B-H. Tok, Royal Society of Chemistry (2009).
- A. Carbonaro, H. Huang, L. A. Godley, and L. L. Sohn, “Cell Characterization Using a Protein-Functionalized Pore”, Lab Chip 9, 1478-1485 (2008).
- A. Shamloo, N. Ma, M.-m. Poo, L.L. Sohn, S.C. Heilshorn, “Endothelial cell chemotaxis in a shear stress free microfluidic device”, Lab Chip 8, 1292-1299.
- A. Carbonaro, L. A. Godley, and L. L. Sohn, “Cell Characterization Using Protein-Functionalized Pores”, The Proceedings of µTAS 2007 Conference (2007), eds. J. –L. Viovy, P. Tabeling, S Descroix, and L. Malaquin, The Chemical and Biological Microsystems Society (2007).
- A. Thupil, M-m. Poo, and L. L. Sohn, “Studying Cell Chemotaxis Using a Microfluidic-Gradient Generator”, The Proceedings of µTAS 2007 Conference (2007), eds. J. –L. Viovy, P. Tabeling, S Descroix, and L. Malaquin, The Chemical and Biological Microsystems Society (2007).
- L. L. Sohn, J. L. Herberg, B. D. Harteneck, D. R. Myers, and J. A. Liddle, “Fabrication of an On-Chip NMR Microfluidics Device”, in the Tenth International Conference on Miniaturized Systems for Chemistry and Life Sciences, Japan Academic Association (2006).
- A. Carbonaro, L. A. Godley, and L. L. Sohn, “The NanoCytometer: A New Method of Cell Detection Performed at the Nanoscale”, in the Tenth International Conference on Miniaturized Systems for Chemistry and Life Sciences, Japan Academic Association (2006).
- A. Carbonaro and L. L. Sohn, “A Resistive Pulse Sensor for Multianalyte Detection”, Lab Chip 5, 1155-1160, 2005.
- B. K. Weis, L. L. Sohn, et al., “Personalized Exposure Assessment: Enabling Population-Based Environmental Research”, Environmental Health Perspectives 113, 840-848.
- O. A. Saleh and L. L. Sohn, “An On-Chip Artificial Pore for Molecular Sensing”, in the Handbook of BioMEMS and Biomedical Nanotechnology, eds. R. Bashir and S. Wereley, Kluwer Academic Press (2005).
- S. W. Mohanty, L. L. Sohn, and D. J. Beebe, “Hybrid Polymer/Thin-Film Impedance System for Label-Free Monitoring of Cells”, to appear in the 26th Annual International Conference, IEEE Engineering in Medicine and Biology Society, (2004).
- O. A. Saleh and L. L. Sohn, “Biological Sensing with an On-Chip Resistive Pulse Analyzer”, to appear in the 26th Annual International Conference, IEEE Engineering in Medicine and Biology Society, (2004).
- I. H. Chan, A. Carbonaro, and L. L. Sohn, “Artificial Pores for Performing Immunoassays”, International Conference on Miniaturized Chemical and Biochemical Analysis Systems ,Kluwer Academic Publishers (2004).
- T. Messina, L. N. Dunkleberger, G. A. Mensing, A. S. Kalmbach, R. Weiss, D. Beebe, and L. L. Sohn, “A Novel High-Frequency Sensor for Biological Discrimination”, in the International Conference on Miniaturized Chemical and Biochemical Analysis Systems, Kluwer Academic Publishers (2003).
- O. A. Saleh and L. L. Sohn, “Direct Detection of Antibody-Antigen Binding Using an On-Chip Artificial Pore”, Proc. Natl. Acad. Sci. 100, 820-824 (2003).
- O. A. Saleh and L. L. Sohn, An Artificial Nanopore for Molecular Sensing”, NanoLetters 3, 37-38 (2003).
- O. A. Saleh and L. L. Sohn, “Correcting Off-Axis Effects in an On-chip Resistive Pulse Analyzer”, Rev. Sci. Inst. 73, 4396-4398 (2002).
- S. Stupp, L. L. Sohn et al., “Small Wonders, Endless Frontiers: A Review of the National Nanotechnology Initiative”, National Research Council & National Academy of Engineering (2002).
- Bockrath, N. Markovic, A. Shepard, M. Tinkham, L. Gurevich, L. P. Kouwenhoven, M. W. Wu, and L. L. Sohn, “Scanned Conductance Microscopy of Carbon Nanotubes and λ-DNA”, NanoLetters, 2, 187-190, 2002.
- G. R. Facer, D. A. Notterman, and L. L. Sohn, “Electronic Biosensing”, appears as an invited chapter in the National Institutes of Environmental Health Sciences, National Institutes of Health, Biomarkers of Environmentally Associated Disease, eds. S. H. Wilson and W. A. Suk, CRC Press, 527-548 (2002).
- O. A. Saleh and L. L. Sohn, “Quantitative Sensing of Sub-Micron Colloids Using a Microchip Coulter Counter”, Rev. Sci. Inst. 72, 4449 (2001).
- O. A. Saleh and L. L. Sohn, “A Quantitative Nanoscale Coulter Counter”, in the Fifth International Conference on Miniaturized Chemical and Biochemical Analysis Systems, Kluwer Academic Publishers (2001).
- D. C. G. Klein, L. Gurevich, J. W. Janssen, L. P. Kouwenhoven, J. D. Carbeck, and L. L. Sohn, “Ordered Stretching of Single Molecules of DNA”, Appl. Phys. Lett. 78, 2396 (2001).
- G. R. Facer, D. A. Notterman, and L. L. Sohn, “Dielectric Spectroscopy for Bioanalysis: 40 Hz to 26.5 GHz in a Microfabricated Waveguide”, Appl. Phys. Lett. 78, 996 (2001).
- O. A. Saleh and L. l. Sohn, “A Resistive Sensing Device for Biological Solutions”, Biophysical Journal 80 (1): 637, Part 2 Jan 2001.
- G. R. Facer, D. A. Notterman, and L. L. Sohn, “Electronic Characterization of Biological Fluid Samples: 40 Hz to 30 GHz”, Biophysical Journal 80 (1): 652, Part 2 Jan 2001.
- L. L. Sohn, O. A. Saleh, G. R. Facer, A. Beavis, R. S. Allan, and D. A. Notterman, “Capacitance Cytometry: Measuring Biological Cells One-by-One”, Biophysical Journal 80 (1): 639, Part 2 Jan 2001.
- L. L. Sohn, O. A. Saleh, G. R. Facer, A. Beavis, R. S. Allan, and D. A. Notterman, “Capacitance Cytometry: Measuring Biological Cells One-by-One”, Proc. Natl. Acad. Sci. 97, 10687 (2000).
- Mingshaw W. Wu and Lydia L. Sohn, “Nanometer-scale Copper Electrodeposition from an On-Chip Source”, IEEE Electron Device Letters 21, 277 (2000).
- L. L. Sohn, “Quantum Leap for Electronics”, Nature 394, 131 (1998).
- O. B. Bakajin, J. P. Brody, J. Chou, S. S. Chan, T. Duke, J. Knight, L. Sohn, A. Vishwanath, R. H. Austin, and E. C. Cox, “Polymer Dynamics and Fluid Flow in Microfabricated Devices”, Proc. SPIE 3258, 100 (1998).
- “Mesoscopic Electron Transport”, NATO ASI Series, Vol. E 345, eds. L. L. Sohn, L. P. Kouwenhoven, and G. Schön (Boston, Kluwer Academic Publishers 1997).
- L. Sohn, C. T. Black, M. Eriksson, M. Crommie, and H. Hess, “Scanning Probe Microscopes and Their Applications”, in Mesoscopic Electron Transport, NATO ASI Series, Vol. E 345, eds. L. L. Sohn, L. P. Kouwenhoven, and G. Schön (Boston, Kluwer Academic Publishers (1997).
- Y. Xia, J. McClelland, R. Gupta, D. Qin, X. Zhao, L. L. Sohn, R. Celotta, and G. M. Whitesides, “Replica Molding Using Polymeric Materials: A Practical Step Toward Nanomanufacturing”, Adv. Mater. 9, 147 (1997).
- L. L. Sohn and R. L. Willett, “Fabrication of Metallic Nanostructures with an Atomic Force Microscope”, Surf. Sci. 362, 874 (1996).
- L. L. Sohn and R. L. Willett, “Fabrication of Nanostructures Using Atomic-Force-Microscope Based Lithography”, Appl. Phys. Lett. 67, 1552 (1995).
- T. J. Shaw, M. J. Ferrari, L. L. Sohn, D. H. Lee, M. Tinkham, and J. Clarke, “Magnetic Flux Noise Study of the KTB Transition in an Overdamped Josephson-Junction Array”, Phys. Rev. Lett. 76, 2551 (1996).
- L. L. Sohn, A. Pinczuk, B. S. Dennis, L. N. Pfeiffer, K. W. West, and L. Brey, “Dispersive Collective Excitation Modes in the Quantum Hall Regime”, Solid State Commun. 93, 897 (1995).
- L. L. Sohn and M. Octavio, “Half-integer steps in single-plaquette Josephson-junction arrays”, Rapid Communications, Physical Review B49, 9236 (1994).
- W. J. Elion, J. J. Wachters, L. L. Sohn, and J. E. Mooij, “The Aharonov-Casher Effect for Vortices in Josephson-Junction Arrays”, Physica B, 497 (1994).
- L. L. Sohn, J. Romijn, E. v.d. Drift, W. J. Elion, and J. E. Mooij, “Fabrication of a Quasi-3-Dimensional Josephson-Junction Array”, Physica B, 125 (1994).
- W. J. Elion, J. J. Wachters, L. L. Sohn, and J. E. Mooij, “Quantum Interference of Vortices in Josephson-Junction Arrays”, Physica B, 1001 (1994).
- L. L. Sohn, J. J. Wachters, U. Geigenmuller, W. J. Elion, and J. E. Mooij, “Static and Dynamic Properties of Vortices in Small Josephson-Junction Arrays”, Physica B, 1059 (1994).
- W. J. Elion, J. J. Wachters, L. L. Sohn, and J. E. Mooij, “Observation of the Aharonov-Casher effect of vortices in Josephson-junction arrays”, Phys. Rev. Lett. 71, 461 (1993).
- L. L. Sohn, M. T. Tuominen, M. S. Rzchowski, J. U. Free, and M. Tinkham, “AC and DC properties of Josephson junction arrays with long-range interaction”, Physical Review B47, 975 (1993).
- L. L. Sohn, M. S. Rzchowski, J. U. Free, and M. Tinkham, “Phase transitions in Josephson junction arrays with long-range interaction”, Physical Review B47, 967 (1993).
- L. L. Sohn, M. S. Rzchowski, J. U. Free, S. P. Benz, M. Tinkham and C. J. Lobb, “Absence of fractional giant Shapiro steps in diagonal Josephson-junction arrays”, Rapid Communications, Physical Review B44, 925 (1991).
- M. S. Rzchowski, L. L. Sohn, and M. Tinkham, “Frequency Dependence of Shapiro Steps in Josephson-Junction Arrays”, Rapid Communications, Physical Review B43, 8682 (1991).